1 millimeter mm 10-3 m. Conversion Rate Total number of conversions Total number of unique visitors 100.

Dimensional Analysis Boundless Chemistry
Cancel units and perform the math calculations eg multiply divide.

. Roughly 90 of all the mathematics done in beginning chemistry involves using one or more conversion factors. Whether you have miles and need kilometers or you have kilometers and need miles you can use either conversion factor between miles and kilometers namely 1 mi 161 km or 1 km 0621 mi. The appropriate conversion factor is.
We have been using conversion factors throughout most of our lives without realizing it. We use these equivalence statements to create our conversion factors to help us cancel out the unwanted units. Now we need to assign numbers to the question marks in the conversion factor.
Conversion Rate Total number of conversions Total number of sessions 100. You might already know some conversion factors without having to look them up like 12 inches per foot 60 seconds per minute 60 minutes per hour and 24 hours per day. 3 hours and 36 minutes 180 minutes plus 36 minutes 216 minutes.
Using minutes is easier because the end time value will need to be in seconds. If you will be doing a lot. An important aspect is that all conversion factors must equal 1 if you replace one unit with another like this.
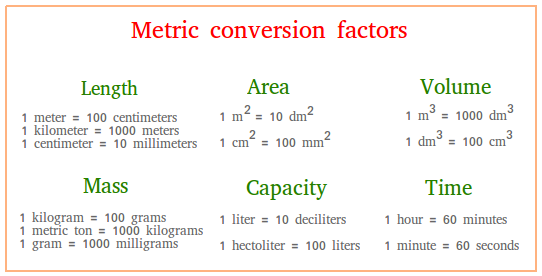
1 kilometer km 1000 meters m 1 meter m 100 centimeter cm 1 centimeter cm 10-2 m. 25g times 1 kg for every 1000 g equals 0025 kg. 470 nF to uF nanofarads to microfarads 10 MF to F megafarads to farads 60 mF to uF millifarads to microfarads 100 picofarad to microfarad pF to μF 2200 uF to farad microfarads to F 10000 pF to uF.
Conversion Rate Total number of conversions Total number of leads 100. 1 m 100 cm. Calculate the volume using these measurements and you get.
Use EXACT conversion factors whenever available. Here are 3 conversion rate formulas to use. For our example of movement on the San Andreas Fault we need to convert 1 km to cm and Myr Mega-years or Million years to years so with the handy guide to metric system prefixes we need three conversion factors.
The conversion factor will be a ratio or fraction. Conversion Factors tutorialdoc Daley 1 10909 Introduction In this tutorial you will learn to use conversion factors to solve a variety of problems that arise in chemistry. 6 pF 2 3.
These are the Desired Units. 1 kg 1000 g 1000 g 1000 g 1 and. However if you say ahh 25 is close enough then the conversion factor only has 2 significant digits.
Identify the unit you want. 60 minutes 1 hour. Now consider the dimensions of the cube in centimeters.
The cube of 1 is 1 the cube of 3 is 27 and the units of length will be cubed to be units of volume Using these facts I get. All 3 of these formulas are valid. For example an inch is defined as 254 cm.
By knowing how many dimes are in a dollar we know that twenty dimes equals two dollars. 22 Unit Conversions in the Field. 21 Unit Conversion and Conversion Factors.
Conversion factors come in a many forms. Arrange the conversion factors so unwanted units cancel. 1 inch in 254 cm.
Identify appropriate unit conversion factor s. It will have the unit we want - meters in this case - in the numerator top and the unit we have - feet - in the denominator bottom. 1 micron μ 10-6 m.
If you use that number it has infinite precision. Multiplication by 1 is what you do whenever you do a problem involving conversion factors. If youre not sure about that cubic-yards and cubic-feet equivalence then use the fact that one yard equals three feet and then cube everything.
In order to use dimensional analysis we must first talk about conversion factors. 1 angstrom A 10-10 m. The conversion ratios are 1 wheelbarrow 6 ft3 and 1 yd3 27 ft3.
1 millimicron mμ 10-9 m. To convert units of area or volume using length measurements square or cube everything in your conversion factor not just the units and everything works out just fine. This volume is much greater than 254 cm 3.
This can be read as. 1 hour 60 minutes. 6 hours agoThe capacitor conversion chart below reveals the equivalents between µF nF and pF in an easy to use table format.
Sep 15 2016 at 327. This is so that the old units will cancel out leaving us with the new units. 1 km 1000 m.
Change 3 hours and 36 minutes to the same units. We all know from elementary school math that if you multiply any quantity by 1 you get the same quantity back. The unit that is used in the denominator is the one to cancels the unit that appears in a numerator.
You know a chain is larger than a foot. Some people call thi. These are the Linking or Ratio Unit s.
Conversion factors allow us to convert from one unit dimes to another dollars. Find the conversion factors that will help you step by step get to the units you want. How do you use Conversion Factors.
You would set up the conversion factor like this. Days are the units we want to get to. Converting from feet to chains should therefore give you a smaller number because the end result will be in chains and each chain contains multiple feet.
Find a conversion factor between the given units and the desired units and write it as an equation. Use what you know about units to check your result. Eromod The conversion factor is only exact if you use that exact conversion factor.
Either equation will work equally well. 1 kg 1000 g is your conversion factor. 25 g 1kg 1000g 0025 kg.
You can do this as many times as you want. Show conversion factors for the example problem. For example 21 2 and 18111 18.
24 hours 1 day. This unit can be hours or minutes.

Useful Conversion Factors And Relationships Chemistry Education Conversion Factors College Chemistry

Converting Units With Conversion Factors Metric System Review Dimensional Analysis Youtube

0 Comments